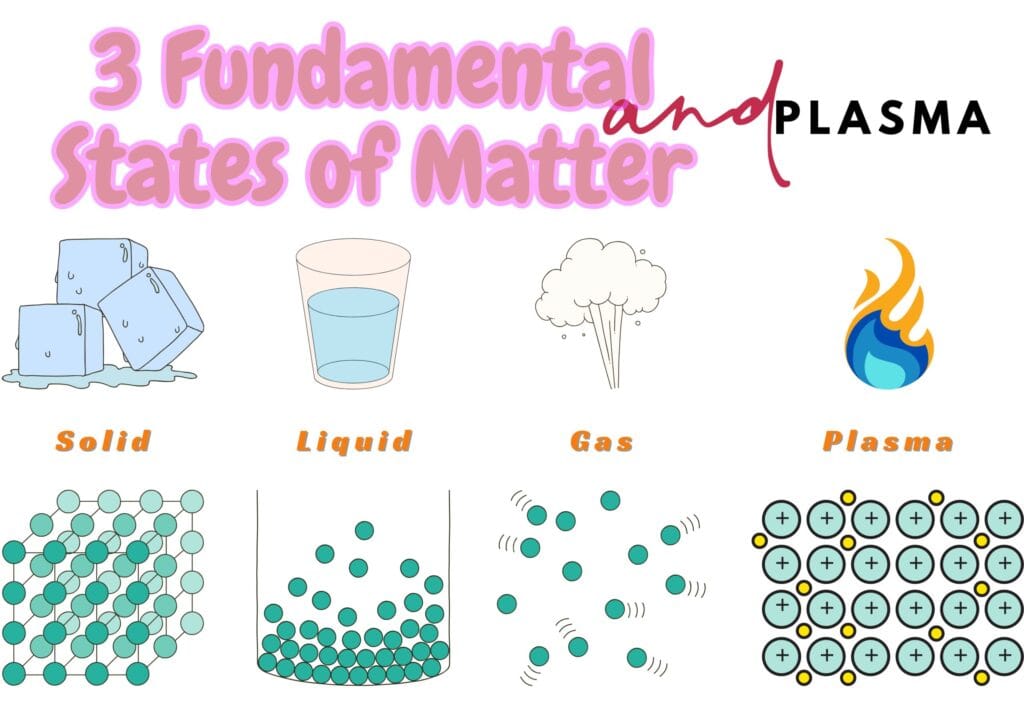
The observable universe gives us smooth access to 3 fundamental states of matter — solid, liquid, and gas — along with plasma. These four states form the core of countless functions and actions in our everyday lives.
Table of Contents
Introduction
Previously, we have talked about matter, the classification of different states of matter, and their types based on the form. We also learnt about the particle model of matter which is a framework to study the behaviour and interaction of different states of matter in our environment.
3 Fundamental States of Matter and Plasma
Here, we shall pursue our quest to understand the matter in depth and learn about the main features of the 4 main states of matter i.e., solids, liquids, gases, and plasma, and answer those questions that may bother the beginners. These questions include;
- How do we characterise the states of matter?
- What are the key properties of these states of matter?
- How does the structure of these states justify their properties?
What Are Solids?
From the laptop, mobile, or tablet in your hand to the Earth beneath you, most things around you are in a solid state. So, take a minute and look around you and tell what other things are in solid state.
Example
Some of the examples of solids are;
- Pen
- Glass
- Rocks
- Jewellery
- Your Body
Characteristics of Solids
How do we know that the things we mentioned are solids? Primarily, solids can be identified by the following features:
- Expand slightly when heated
- Rigid and hard (though some can be soft or flexible)
- Often crystalline (like salt) or amorphous (like glass)
So, anything around you that justifies these three criteria is in solid state.
Structure of Solids
The properties of solids inspire us to understand why solids behave the way they do. For that, we need to look at their internal structure. The key aspects of the internal structure of solids are:
- Particles (atoms or molecules) are tightly packed in a fixed and orderly lattice.
- Strong intermolecular forces keep the particles in place.
- Particles vibrate in place but do not move freely.
So, it is this arrangement of particles that explains why solids behave the way they do. Whether it is a concrete block or a wooden block, the internal particle structure defines its external properties and, consequently, its applications.
2. What are Liquids?
From the water to quench your thirst to the milk that makes you taller and stronger are the liquid states of matter. Can you think about more of such things?
Example
A list of common liquids are listed here:
- Juices
- Honey
- Petrol
- Diesel
- Medicinal Syrup
- Antiseptic Solution
Characteristics of Liquids
Just like solids, liquids also show distinguish characteristic and these are given below:
- Capable of diffusion
- Exhibit viscosity (resistance to flow)
- Surface tension (due to cohesive forces)
If you want to learn more about these three terms, try following simple and safe experiments at home.

Structure of Liquids
The internal structure of liquids (the arrangement of particles) clarifies the reason why the properties of liquids lie between solids and gases. For instance,
- Particles are close together (like solids), but not in a fixed position (like gases).
- Particles have weaker intermolecular forces than solids, which allow particles to move past each other.
- Free mobility of particles gives liquids the ability to flow and take the shape of their container.
3. What are Gases?
Gas is one of the 3 primary states of matter (the 4th being plasma). Like solids and liquids, gases have their own unique characteristics and structural features.
Even if we cannot always see them, we encounter gases every day. For instance, from the air we breathe to the steam that rises from our cup of tea or coffee, gases are all around us.
Example
- Air (a mixture of different gases)
- Carbon dioxide (CO₂)
- Helium in balloons
- Oxygen (O₂)
- Natural gas
- Steam
Characteristics of Gases
- Exert pressure on the walls of their container
- Mix evenly and completely in a container (diffusion)
- Follow gas laws (Boyle’s, Charles’s, etc.)
Structure of Gases
- Particles are far apart and move randomly and rapidly.
- Particles experience negligible intermolecular forces (in ideal gases).
- A large amount of space exists between particles, which explains the compressibility and expansion ability of a gas.
4. What is Plasma?
Plasma is the rarest of the 4 states of matter on the Earth. However, it is found in abundance in stars and interstellar space. On Earth, plasma is mostly studied in research labs and advanced technologies. However, it also appears naturally in certain high-energy conditions. Do you know about those?
Example
- The Sun
- Plasma TVs
- Fluorescent lights
Characteristics of Plasma
- Only visible when excited particles emit light and radiation
- Found in stars, lightning, auroras, neon signs, and fusion reactors
Structure of Plasma
- Plasma consists of ionised gas.
- It behaves differently from gases due to electromagnetic interactions
- High energy breaks the atomic bonds and strips atoms of electrons, forming a mix of positive ions and free electrons.
- The charged particles move freely and yield in the electrical conductivity of plasma, which is affected in EM fields.
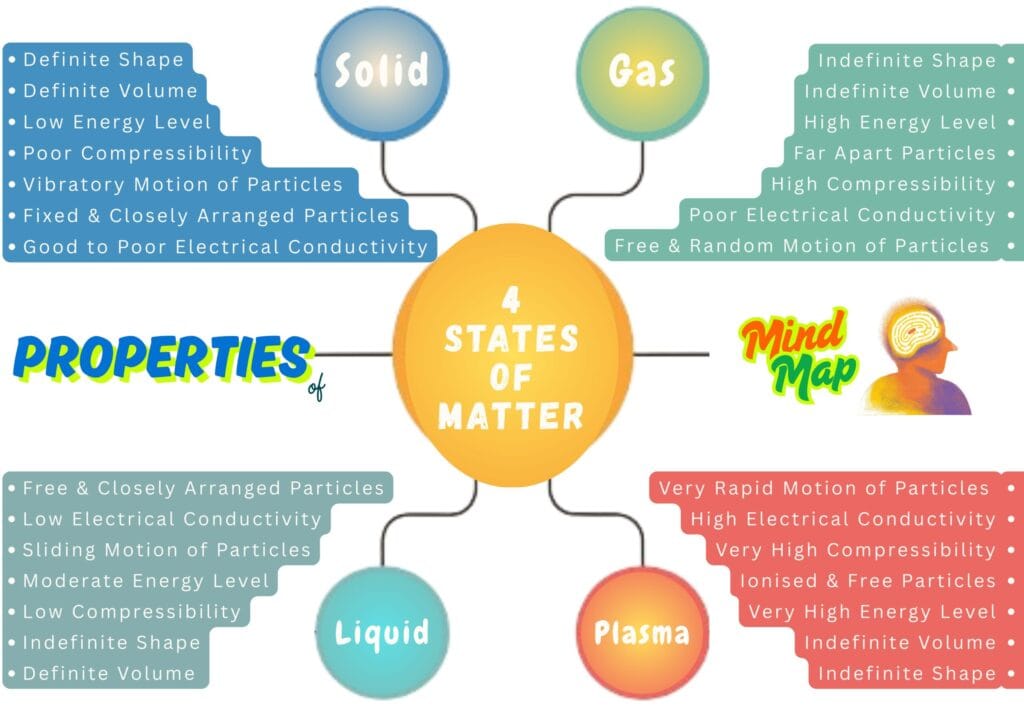
Properties of 4 States of Matter | Solid, Liquid, Gas, and Plasma
Each state of matter has its unique properties, which decide the application of that state of matter. Here, we have listed the key properties of 4 states of matter as a mind map.
Conclusion
The matter is not just a concept from textbooks. It is a source and a proof of enlightenment to understand the universe through curiosity, observation, and experimentation. Learning about the different states of matter does not just expand our knowledge; it helps us improve the way we live and interact with the world.
Whether it is the marvel of engineering, building better technologies, exploring space, or diving into the strange world of quantum mechanics, our understanding of matter matters a lot. It is this clarity that strengthens our progress and pushes us forward as a civilisation.
Frequently Asked Questions (FAQs)
What is the main difference between the structure of solids, liquids, and gases at the particle level?
Solids have tightly packed particles in a fixed lattice, liquids have closely packed particles with free movement, and gases have particles that are far apart and move randomly at high speeds.
Why do solids maintain a definite shape while liquids and gases do not?
Solids maintain a definite shape because of the strong intermolecular forces and fixed particle arrangement, while liquids and gases have weaker forces that allow particles to move freely, adapting to the shape of their container.
How does the internal particle arrangement of liquids allow them to flow but not compress easily?
In liquids, particles are close enough to resist compression but not locked into a fixed position, allowing them to slide over each other and flow freely.
Why do gases expand to fill the entire volume of a container while liquids stay at the bottom?
Gases expand to fill a container because their particles move independently with negligible attraction, whereas liquids are held together by cohesive forces that keep them in a definite volume at the bottom.
How does plasma differ from a gas even though both have free-moving particles?
Plasma differs from gas because its particles are ionised — meaning it consists of positive ions and free electrons — and it reacts strongly to electric and magnetic fields, unlike neutral gas particles.
What role does energy play in changing a solid into a liquid, liquid into a gas, and gas into plasma?
Energy increases particle movement: in solids to liquids (melting), it overcomes rigid bonds; in liquids to gases (vaporisation), it separates particles further; in gases to plasma (ionisation), it strips electrons from atoms.
Why plasma is considered the most abundant state of matter in the universe but rare on Earth?
Plasma is abundant in the universe because stars, including the sun, are made mostly of plasma, but on Earth, the conditions for natural plasma formation (extremely high energy) occur less frequently.
How do the properties of surface tension and viscosity in liquids relate to their particle structure?
Surface tension arises from cohesive forces pulling particles together at the surface, while viscosity depends on how easily particles can slide past one another within the liquid.
In what ways do gases demonstrate the principles of Boyle’s Law and Charles’s Law in real life?
Gases demonstrate Boyle’s Law (pressure inversely proportional to volume) and Charles’s Law (volume directly proportional to temperature) through everyday phenomena like inflating a balloon or a heated airbag expanding.
How does the particle model of matter explain the compressibility differences between solids, liquids, and gases?
The particle model shows that solids and liquids have closely packed particles that resist compression, while gases have widely spaced particles that can be compressed easily by reducing the space between them.
