Mixtures can broadly be classified into three main categories: solutions vs colloids vs suspensions. This classification is based on the size of their constituent particles and how they interact with the solvent,
Table of Contents
Introduction
Every day, we encounter mixtures—whether it is the milk in your breakfast, the air you breathe, or muddy water after a rainstorm. But not all mixtures behave the same way.
In chemistry, mixtures are classified based on two possible criteria:
- Composition of the substance
- Size of the particles and their interaction with the surrounding substance
Understanding the differences between these types is fundamental in various scientific disciplines and everyday life.
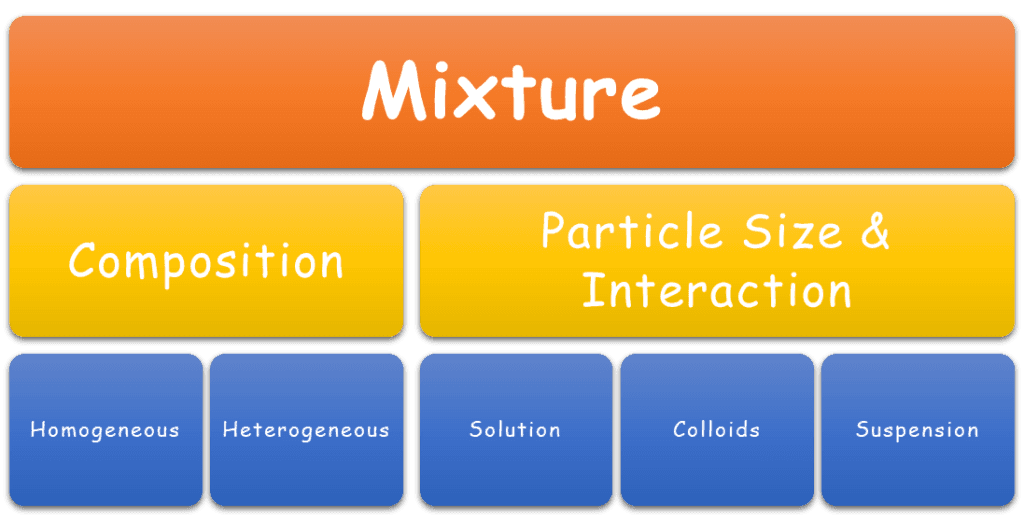
Here, we shall learn how to tell them apart based on the size of particles and their interaction.
In the world of chemistry, mixtures are all around us.
1. What is a Solution?
A solution is a homogeneous mixture where the solute particles are completely and uniformly dissolved within the solvent.
Characteristics of Solutions
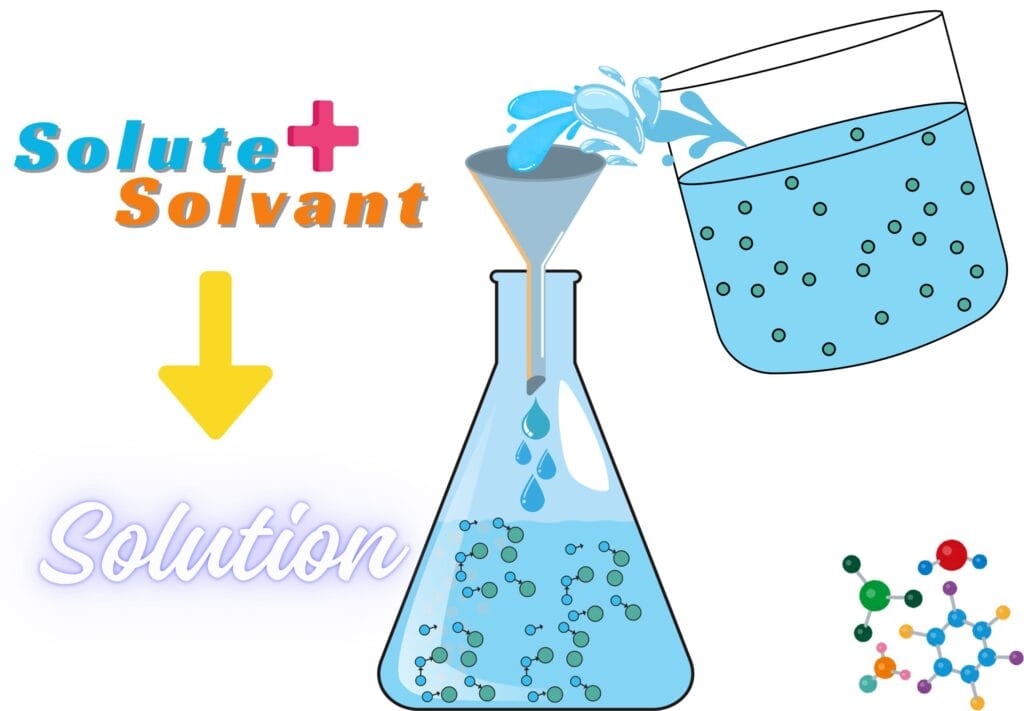
i. Particle Size
The solute particles in a true solution are extremely small. They are typically of the order of 10⁻⁸ cm or less. Due to their minuscule size, they cannot be seen with the naked eye. They exist as molecules or ions.
ii. Homogeneity
Solutions exhibit uniform composition (character) throughout. The individual components of a solution are not visible.
iii. Settling
Solute particles in a true solution will not settle down even if left undisturbed for a long period. They are constantly in motion due to the kinetic energy of the solvent molecules.
iv. Filterability
The small size of solute particles enables it to easily pass through the pores of a filter paper, leaving no residue.
v. Tyndall Effect
Tyndall effect (scattering of light beam passing through the solution) is absent in a true solution. The path of light will not be visible within the solution.
vi. Forces of Interaction
Strong intermolecular forces exist between the solute and solvent particles. A true solution can only be formed if:
![]()
Example
- Sugar dissolved in water (sugar solution)
- Salt dissolved in water (saline solution)
- Copper sulphate dissolved in water
- A drop of ink mixed in water
- Air (a gaseous solution)
2. What is a Colloidal Solution?
Colloidal solutions, often referred to as colloids or false solutions, are mixtures where the solute particles are larger than those in true solutions but not large enough to settle out under gravity.
Characteristics of Colloids
i. Particle Size
The particles in a colloidal solution are larger than those in true solutions. However, generally, it is still too small to be seen with the naked eye. Their size ranges from 10⁻⁷ to 10⁻⁵ cm in diameter.
ii. Apparent Homogeneity
A colloid appears to be homogeneous to the naked eye, but it is a heterogeneous mixture at a microscopic level.
iii. Settling
Colloidal particles are quite stable. They do not settle down easily even when left undisturbed for a long time.
This stability is due to factors like Brownian motion and charge repulsion between the colloidal particles.
Brownian motion is the random, zigzag movement of tiny particles. It is caused by constant collisions with surrounding molecules.
This motion helps keep colloidal particles suspended, preventing them from settling.
iv. Filterability
Colloidal particles are often too large to pass through ordinary filter paper. They may require ultrafilters for separation.
v. Tyndall Effect
Tyndall effect is the characteristic phenomenon of colloids.
When a beam of light is passed through colloids, the relatively larger particles scatter the light. This makes the path of the light beam visible within the colloid.
This is a key way to distinguish colloids from true solutions.
Examples
- Albumin (egg white)
- Starch solution
- Soap solutions
- Toothpaste
- Smoke
- Blood
- Milk
- Jelly
- Fog
- Ink
3. What is a Suspension?
A suspension is a heterogeneous mixture in which the solute particles are large enough to be seen with the naked eye and do not dissolve in the solvent.
Characteristics of Suspensions
i. Particle Size
The particles in a suspension are the largest among the three types. Their diameters are larger than 10⁻⁵ cm. They are big enough to be visible without any magnification.
ii. Heterogeneity
Suspensions are distinctly heterogeneous. Its particles are dispersed throughout the medium and can easily be seen.
iii. Settling
The particles in a suspension are unstable. They will settle down under the influence of gravity if left undisturbed for some time. This sedimentation is a key characteristic of suspensions.
iv. Filterability
Due to their large size, solute particles in a suspension cannot pass through a filter paper. They can easily be separated by filtration, leaving a residue.
v. Tyndall Effect
Suspensions are opaque because of their large particles that block or absorb light. It makes it hard for the light to pass through or show a clear Tyndall effect.
Examples
- Milk of magnesia (suspension of magnesium hydroxide in water)
- Chalk in water (milky suspension)
- Paints (before settling)
- Sand in water
- Muddy water
Key Differences between Solutions vs Colloids vs Suspensions
The key differences between solutions, colloids, and suspensions are listed here:
| Feature | Solution | Colloid | Suspension |
| Particle Size | < 10⁻⁸ cm | 10⁻⁷ to 10⁻⁵ cm | > 10⁻⁵ cm |
| Nature of Particle | molecules or ions | aggregates of atoms/ions/molecules | large particles |
| Homogeneity | Homogeneous | Appears homogeneous, but heterogeneous | Heterogeneous |
| Visibility | Particles not visible to the naked eye | Particles not visible to the naked eye | Particles visible to the naked eye |
| Settling | No settling | No settling (stable) | Particles settle down after some time |
| Filterability | Passes easily through filter paper | May pass through ordinary filter paper, but not easily | Cannot pass through filter paper (residue formed) |
| Scattering of Light | Does not scatter light (no Tyndall effect) | Scatters light (exhibits Tyndall effect) | Light is blocked and difficult to pass |
| Forces of Interaction | Strong solute-solvent attraction keeps particles mixed | Moderate forces; stability aided by charge and motion | Weak interactions; particles easily separate |
| Examples | sugar dissolved in water and Salt in water | Milk and Fog | oil and water and shake-before-use medicines |
If you need more clarity on the topic, download the file.
Conclusion
Understanding the distinctions between solutions, colloids, and suspensions is crucial. They enhance our comprehension of various chemical and physical processes.
This classification helps us analyse and categorise the diverse mixtures we encounter daily. By focusing on particle size, homogeneity, settling behaviour, filterability, and the Tyndall effect, we can effectively differentiate between these fundamental types of mixtures.
Frequently Asked Questions (FAQs)
How can I easily identify whether a mixture is a solution, colloid, or suspension?
You can identify them based on visibility, settling, and light scattering:
- Solutions are clear and stable, with no visible particles.
- Colloids scatter light (Tyndall effect) but do not settle.
- Suspensions have visible particles that settle over time and can be filtered.
What is the Tyndall effect, and why does it not occur in true solutions?
The Tyndall effect is the scattering of light by particles in a mixture. It occurs in colloids because their particle size is large enough to scatter light.
In true solutions, particles are too small to affect the light beam, so the effect is absent. In suspension, the particle blocks the path of light and absorbs it.
Can a substance change from a solution to a suspension or colloid?
Yes!
Depending on particle size, concentration, or chemical changes, a mixture can go through a transition.
- Increasing the concentration may convert a solution into a colloid or suspension.
- Aggregation of solute particles can also change the mixture type.
Why colloids are considered “false solutions”?
Colloids appear homogeneous like true solutions, but under a microscope, their particles are heterogeneously dispersed. This deceptive appearance gives them the name “false solutions.”
Are milk and fog solutions, colloids, or suspensions?
Both milk and fog are colloids.
- Milk is an emulsion (liquid in liquid).
- Fog is an aerosol (liquid in gas).
They scatter light and do not settle (a typical behaviour colloidal).

