Understanding the difference between atmospheric pressure and hydrostatic pressure and the ways of measurement of atmospheric pressure and hydrostatic pressure is crucial in physics.
Table of Contents
Introduction
Pressure is a fundamental concept in physics. From the weather we experience to how we breathe, it affects our daily lives in ways we often do not notice
Tail a Tale | The Science of Pressure
Far from both mountains and seas, in a quiet desert oasis, lived Mr. I. One day, he decided to travel around the world and experience what it is like to ride in the sky and dive beneath the sea. As soon as he took off in an airplane and later explored the deep ocean, he noticed that he felt different in both places.

Why does this happen?
It all comes down to air and water pressure. In an airplane, the air pressure is much lower than on the ground due to lesser oxygen in each breath. This can make breathing feel harder. The rapid change in altitude can also cause our ears to pop as our body tries to adjust to the pressure difference.
Similarly, deep under the sea, water pressure increases with depth. It is because the water molecules keep on pressing more heavily against the body and affecting how we feel and breathe.
The same kind of pressure change can be felt in confined spaces like caves or elevators. For instance, in a fast elevator, our ears might pop due to quick pressure shifts, even though we are only moving a short distance.
In all these cases, our bodies are reacting to changes in pressure, which affect how we breathe and feel.
What is Pressure?
Pressure is defined as,
“the force exerted per unit area.”
Mathematical Formulation
Mathematically, it is given by the formula;
![]()
Here,
![]()
![]()
![]()
For details about pressure, click here.
What is Atmospheric Pressure?
Atmospheric pressure is the pressure exerted by the weight of air in the atmosphere of Earth. It acts in all directions and varies with altitude.
Evidence of Atmospheric Pressure
There are 3 simple physical ways to prove the existence of atmospheric pressure.

Crushed Can Experiment
Take a water-filled can and heat it till steam is produced. Now, seal the can properly and cool it with cold water. This will result in the crushing of the can.
The reason it happened is, that the air inside the can contracts rapidly which creates low pressure inside the can. Meanwhile, the atmospheric pressure outside remains high. The unbalanced pressure crushes the can inward.
This demonstrates the existence of atmospheric pressure.
Suction Cups
Consider a suction cup pressed against a smooth surface. It causes most of the air between the cup and the surface to be pushed out. The higher atmospheric pressure outside the cup then holds it firmly against the surface.
Drinking Through a Straw
When we suck juice through a straw, we pull some of the air out of the straw. This makes the pressure inside the straw lower. The air around us (called atmospheric pressure) then pushes down on the juice in the cup. It helps push the juice up the straw and into your mouth. It is like the air is helping us to take a sip.
Factors Affecting Atmospheric Pressure
- Altitude
Pressure decreases with height. It is because the air pressure drops as we go higher. At sea level, pressure is at its maximum value. Going up just 5 km, it will drop to 55 kPa, and climbing to 30 km further, it will be barely 1 kPa.
This pressure drop is actually how altimeters (used in planes and GPS devices) estimate altitude.
- Temperature
Warm air is lighter, reducing pressure.
- Humidity
Moist air weighs less than dry air, lowering pressure.

Measurement of Atmospheric Pressure
For the measurement of the atmospheric pressure a barometer is used which works on the pressure difference.
What is a Barometer?
A barometer is an instrument that measures atmospheric pressure.
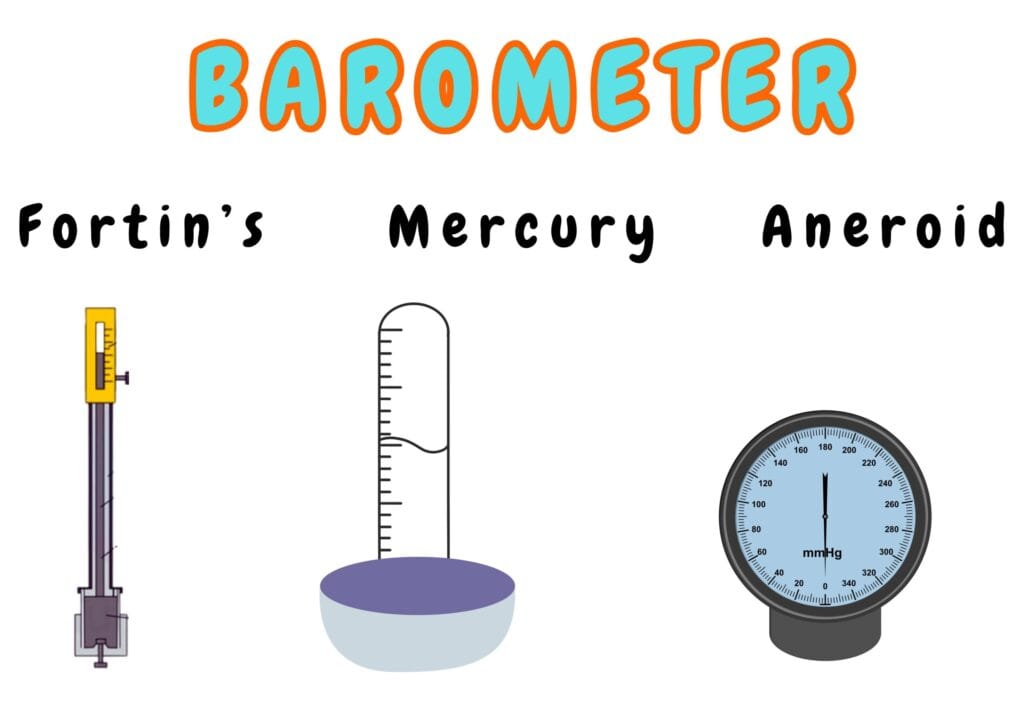
Types of Barometers
- Mercury Barometer
- Fortin’s Barometer
- Aneroid Barometer
Parts of a Mercury Barometer
- Mercury-filled glass tube sealed at one end
- Mercury reservoir
- Calibrated scale
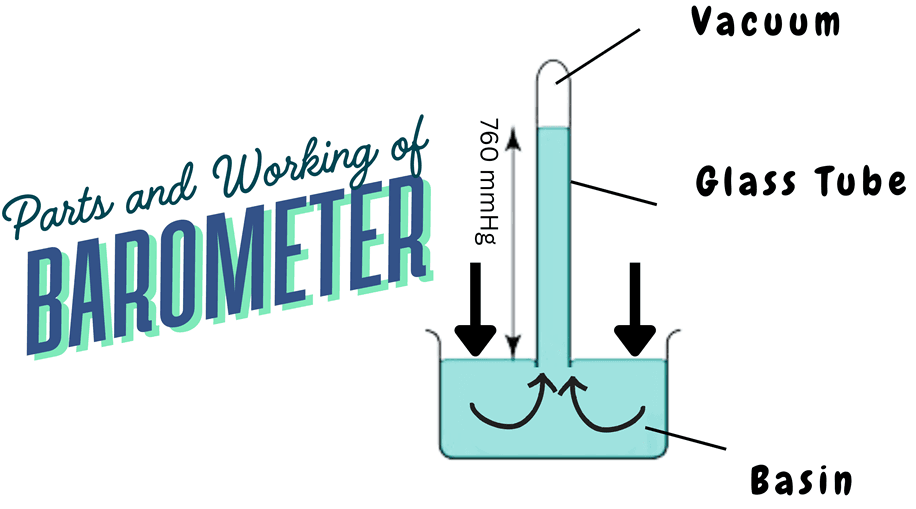
How Does It Work?
1. A long glass tube which is sealed at one end is filled with mercury
2. The tube is inverted into a mercury-filled basin.
3. The mercury in the tube falls slightly, creating a vacuum at the top.
4. The mercury column stabilizes at a height where the weight of the mercury column balances the atmospheric pressure pressing on the basin.
5. The height of the mercury column gives a direct measure of atmospheric pressure which is typically around 760 mmHg at sea level.
6. This reading is equivalent to one atmosphere (1 atm), or approximately 101.3 kilopascals (kPa).
Water in a Barometer
Water cannot be used in a barometer because it has low density. To balance atmospheric pressure, it would need to rise to a very great height, meaning a longer glass tube. This makes water impractical for use in a standard-sized barometer.
It can also be proven mathematically. For instance, we know that for fluids;
![]()
For the mercury column;
![]()
For the water column;
![]()
Comparing the two we get;
![]()
![]()
![]()
Here,
![]()
![]()
Hence,
![]()
![]()
This confirms that a water barometer would need over 10 meters tall column. That is why mercury is used instead of water.
What is Hydrostatic Pressure?
Hydrostatic pressure is the pressure exerted by a fluid at rest due to the force of gravity. It increases with depth in the fluid.
Evidence of Hydrostatic Pressure
The presence of hydrostatic pressure can be seen in 3 simple ways.
Water Jet Experiment
Water flows faster from holes lower in a container, indicating higher pressure at depth.
Dam Design
Thicker bases in a dam show that deeper water exerts more pressure.
Diving with a Balloon
Holding a balloon underwater as we descend, squishes the balloon.
Factors Affecting Hydrostatic Pressure
The pressure due to fluids is given by;
![]()
Here are the factors that influence the hydrostatic pressure.
Greater depth equals higher pressure.
Denser fluids exert more pressure.
More gravity means more pressure.
Measurement of Hydrostatic Pressure
Hydrostatic pressure is typically measured using a manometer.
What is a Manometer?
A manometer is an instrument used to measure the pressure of a gas or liquid.
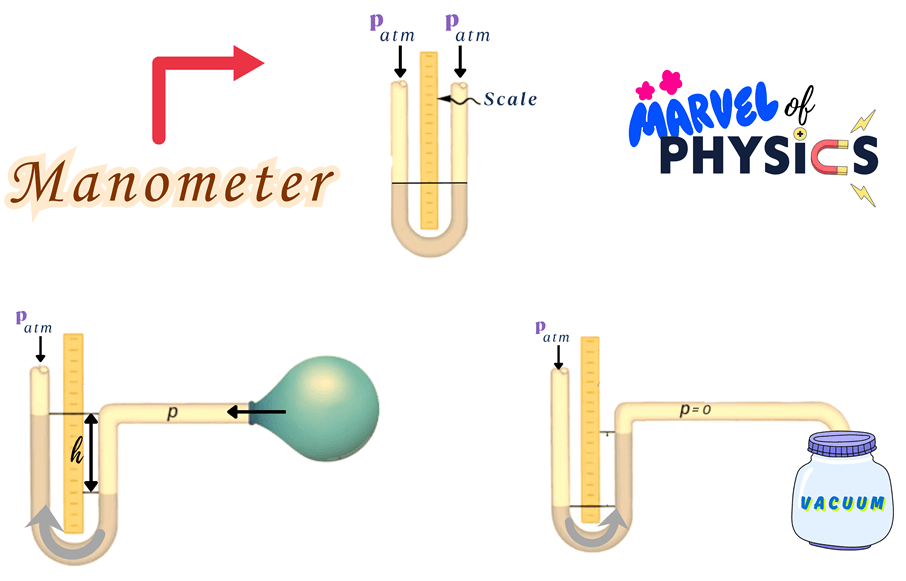
What Parts Is It Made of?
- U-shaped glass tube
- Liquid (usually mercury, water, or oil)
- Graduated scale for measurement
- Connection points to a gas or fluid system

How Does It Work?
1. One end of the manometer is attached to the fluid or gas source.
2. The other end is either open to the atmosphere or sealed, depending on the type.
3. Pressure from the system causes the liquid inside to move, creating a difference in levels.
4. The difference in liquid levels in the U-tube indicates the pressure.
5. Pressure is calculated based on the height difference, using ![]() .
.
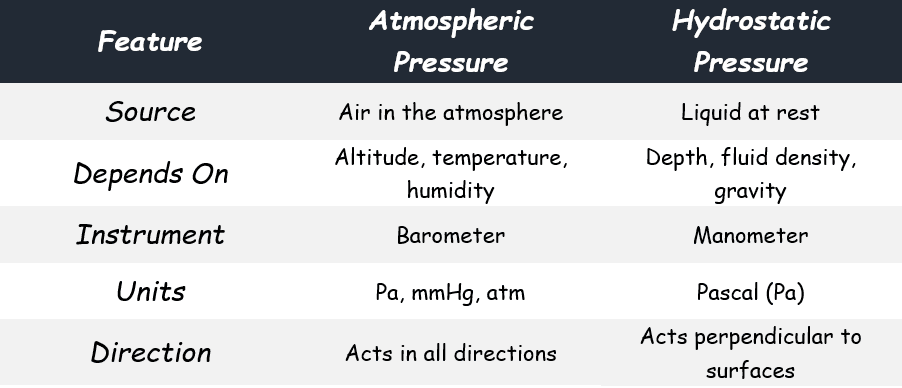
Difference between Atmospheric Pressure and Hydrostatic Pressure
Conclusion
Both atmospheric and hydrostatic pressures are essential concepts in understanding the physical world around us. Atmospheric pressure, exerted by the air around us, influences phenomena from weather patterns to simple activities like drinking through a straw.
Hydrostatic pressure, on the other hand, arises from fluids at rest. It varies with depth and affects everything from dam construction to how we feel underwater.
Understanding these pressures not only enhances our grasp of physics but also helps explain many natural and practical events in our lives.
Frequently Asked Questions
Show that atmosphere exerts pressure.
The atmosphere exerts pressure. This pressure is due to the weight of air molecules pressing down on the surface of the Earth. This is evident in daily life, like when a suction cup sticks to a wall or when a straw pulls up juice.
What is a barometer?
A barometer is an instrument used to measure atmospheric pressure. It helps in predicting weather changes based on pressure variations.
Why water is not suitable to be used in a barometer? What should be the approximate length of a glass tube to construct a water barometer?
Water, in comparison to the mercury, is not a suitable fluid for a barometer. The reason is that it has a low density and requires an impractically long column. So, a mercury barometer is used instead due to its higher density.
The length of the glass tube can be found by using the ratios of height and the density of two fluids i.e. mercury and water.
![]()
Here,
![]()
![]()
Hence,
![]()
![]()
![]()
![]()
What makes a sucker pressed on a smooth wall sticks to it?
When a sucker is pressed, air is removed creating a partial vacuum inside. Atmospheric pressure on the outside holds it tightly against the wall.
What does it mean when the atmospheric pressure at a place falls suddenly? Also, what changes are expected in weather if the barometer reading shows a sudden increase?
A sudden fall in atmospheric pressure usually signals bad weather. It often indicates an approaching storm or low-pressure system.
Similarly, a sudden increase in barometer readings suggests that good weather is on the way. It indicates a high-pressure system bringing clear skies.
Explain that atmosphere exerts pressure. What are its applications? Give at least three examples.
Atmospheric pressure is the force exerted by air on surfaces due to the weight of air above. Applications include suction cups, drinking through straws, and operating syringes.
Describe the working and applications of a simple mercury barometer and a manometer.
A mercury barometer measures atmospheric pressure using a column of mercury that balances against the air pressure. A manometer measures the pressure of a fluid in closed containers and is used in labs and industries.
The end of a glass tube used in a simple barometer is not properly sealed, some leak is present. What will be its effect? Also, if some air remains trapped within the top of the mercury column of the barometer which is supposed to be vacuum, how would it affect the height of the mercury column?
If there is a leak, air enters the tube and affects the vacuum. This causes incorrect readings as the mercury column will be lower than the expected height.
Just like a leak, trapped air also increases internal pressure and reduces the height of the mercury column. This results in an underestimation of atmospheric pressure.
What is the basic principle used to measure the atmospheric pressure by a simple mercury barometer?
The principle is that atmospheric pressure balances the weight of a mercury column. The height of the column reflects the atmospheric pressure at a location.
Why does the atmospheric pressure vary with height?
Atmospheric pressure decreases with height because there is less air above exerting downward force. Higher altitudes have thinner air and lower pressure.
