Knowing about mixtures and 2 types of mixtures is foundational in learning chemistry and the behaviour of matter in our daily lives.
Table of Contents
Introduction
The toothpaste you brush with, the jam on your breakfast toast, the pizza you savour, the ice cream you enjoy, the soil beneath your feet, the rocks in the mountains, and even the air you breathe—all are mixtures. These everyday examples highlight just how common mixtures are in our lives.
But what exactly is a mixture, and why is it important in chemistry and everyday life? Here you shall learn about the fundamentals of mixtures, their types, and how they impact our world.
What Is a Mixture?
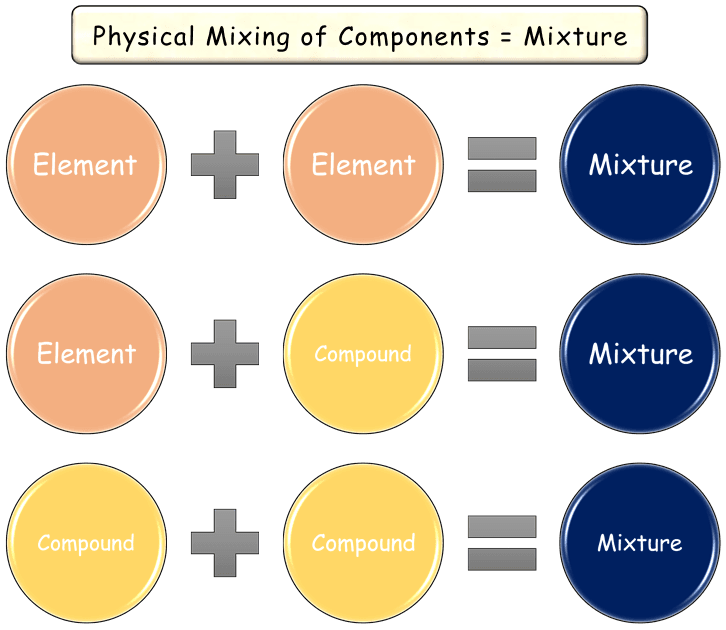
A mixture can be defined as:
“A mixture is an impure form of a substance, obtained by the physical combination of two or more substances—elements or compounds—without a fixed ratio.”
There are 3 key points to understand about mixtures:
i. Mixtures are impure matter, unlike elements and compounds.
ii. Its components are combined physically—meaning no chemical bonding is present.
iii. The components can be mixed in any proportion, without following a specific ratio.
Key Characteristics of Mixtures
- No Fixed Proportion of Components
The substances in a mixture can be combined in any proportion.
Example
If you mix water and juice, you can add more water or more juice, and it is still a juice mix!
- Retention of Identity of Each Substance
Each component in a mixture retains its original chemical properties.
Example
If you mix rice and beans, the rice is still rice and the beans are still beans.
- Separation of Components by Physical Means
The components of a mixture can be separated using physical methods, as no new chemical bonds are formed.
Examples
- Filtration
- Distillation
- Magnetisation
Types of Mixtures
Mixtures fall into two broad categories based on their composition and appearance.
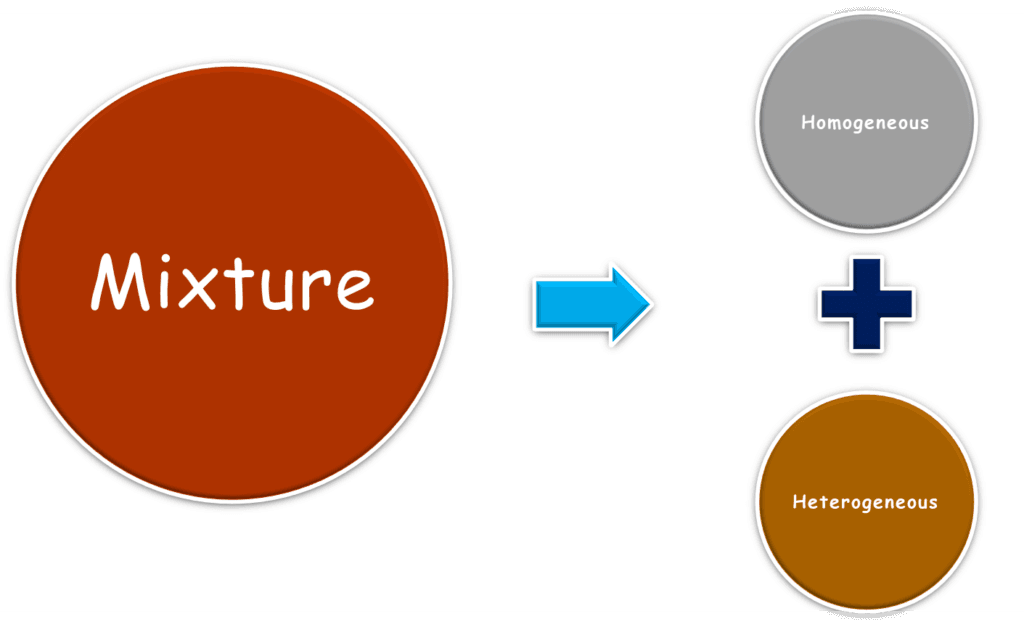
1. Homogeneous Mixtures
In a homogeneous mixture, the composition remains uniform throughout.
You cannot distinguish the individual components, and every sample from the mixture is the same.
Examples
- Brass
An alloy of copper and zinc, with an even distribution of metals.
- Air
A consistent blend of nitrogen, oxygen, carbon dioxide, and trace gases.
- Sea Water
The sea water is full of minerals and yet appears as a single-phase solution.
2. Heterogeneous Mixtures
A heterogeneous mixture exhibits a non-uniform composition throughout.
Its components are often visible and different parts of the mixture may have different properties.
Examples
- Granite
A rock with visibly distinct mineral grains.
- Soil
Contains sand, silt, clay, organic matter, water, and air.
- Milk
It is a colloidal dispersion of fats and proteins in water. It appears uniform but is technically heterogeneous.
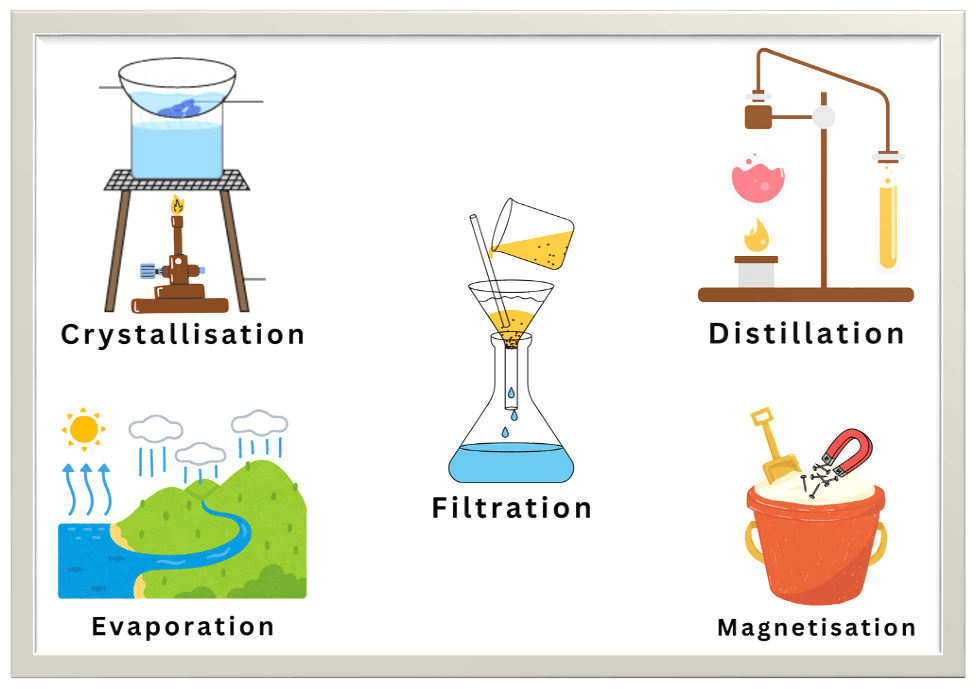
Methods of Separation
Since mixtures do not involve chemical bonding, their components can be separated using physical techniques. These techniques include:
- Filtration
It removes insoluble solids from liquids (e.g., tea leaves from tea).
- Crystallisation
It forms pure solid crystals from a solution (e.g., sugar crystals).
- Distillation
It separates liquids based on boiling points (e.g., alcohol from water).
- Magnetisation
It separates magnetic from non-magnetic materials (e.g., iron from sand).
- Evaporation
It recovers dissolved solids by evaporating the liquid (e.g., salt from seawater).
Mixtures in Daily Life
Mixtures in the environment not only enhance our understanding of chemistry but also play an important role in our daily lives. They are present from the depth of the oceans to the towering mountains. For instance,
- Air (vital homogeneous mixture, essential for life)
- Milk (nutritious colloid with water, fats, proteins, and vitamins)
- Tap Water (contains minerals, gases, and sometimes disinfectants, quench our thirst)
- Wood (complex heterogeneous mix of cellulose, lignin, water, and other organic compounds)

Conclusion
Mixtures are a foundational concept in chemistry. They play a central role in understanding the materials around us. Whether you are brewing coffee, inhaling air, or analysing a mineral sample, you are interacting with mixtures.
By learning what they are, we gain a deeper appreciation for the complexity and beauty of the physical world.
Frequently Asked Questions (FAQs)
Write about mixtures and 2 types of mixtures in chemistry.
A mixture is an impure form of matter formed by the physical mixing of two or more substances without any fixed ratio or chemical bonding.
There are two main types:
- Homogeneous mixtures (uniform composition, e.g., air)
- Heterogeneous mixtures (non-uniform composition, e.g., soil)
How are mixtures different from compounds?
Mixtures have no fixed composition and retain the properties of their components. Compounds, unlike mixtures, have a fixed ratio and form new substances with different properties through chemical bonding.
How to distinguish between a homogeneous mixture and a heterogeneous mixture?
Homogeneous mixtures have a uniform composition throughout e.g., air, tap water, etc.
Heterogeneous mixtures show a non-uniform composition throughout e.g., soil, rock, etc.
Can the components of a mixture be separated?
Yes!
The components of a mixture can be separated using physical methods. Some common methods include filtration, distillation, magnetisation, crystallisation, and evaporation.
Why milk is considered a heterogeneous mixture?
Milk appears uniform, but it is a colloidal dispersion of fats and proteins in water, making it technically a heterogeneous mixture.
What does it mean that mixtures have no fixed proportion?
It means the substances in a mixture can be combined in any amount. They are not bound by the amount of substances.
Do components of a mixture lose their individual properties?
No!
Each component in a mixture retains its original chemical properties.
Why are mixtures important in everyday life?
Mixtures are found everywhere—in food, air, water, soil, and materials. Understanding these enables us to appreciate the composition and function of many things in our daily lives.
How can you justify that air is a homogenous mixture? Identify substances present in it.
Air is a homogeneous mixture because it has a uniform composition throughout and its components are not visibly distinguishable. The substances present in the air include:
- Nitrogen (78%)
- Oxygen (21%)
- Argon (~0.93%)
- Carbon dioxide (~0.04%)
- Water vapor and other trace gases
