The chronological study of the evolution of atomic theory provides a historical perspective for understanding the structure of the atom.
Table of Contents
Introduction
The concept of the atom did not emerge overnight, nor was it the result of a single individual’s work. It took thousands of years of curiosity, experimentation, and bold ideas to evolve into the model you know today.
Throughout history, many thinkers contributed to our understanding of the atom, each building upon or revising the ideas of their predecessors.
Here is the chronological journey of how the atomic structure developed over time.
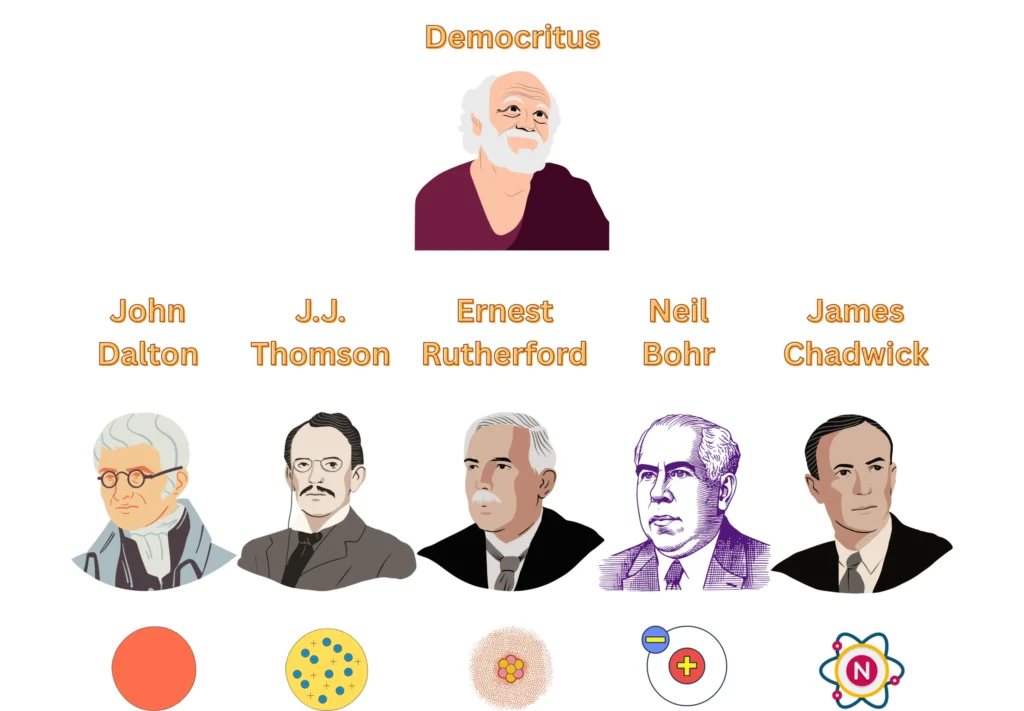
1. Democritus (460 – 370 BC)
Long ago, the Greek philosopher Democritus sat and wondered what would happen if you kept dividing matter into smaller and smaller pieces. His answer was simple yet revolutionary:
“You would reach a particle so small that it could not be cut any further”.
He called these particles atomos, meaning “indivisible.”

Key Ideas
The main idea that Democritus put forth was, all matter is made up of tiny, eternal particles called atoms.
Strengths
Without a doubt, Democritus’s vision was strikingly ahead of its time. For a time when science was more philosophy than experiment, it sure was a radical idea. He was the first to truly imagine matter as granular rather than continuous.
Limitations
Democritus’ theory remained a speculation and was largely ignored for centuries in favour of Aristotle’s view that matter was continuous. Without experiments, he had no way to prove his ideas, which was not possible at his time.
2. The “Lost Centuries” (200 BC – 1000 AD)
After the fall of the Greek world, Europe became less scientifically active, but intellectual traditions thrived elsewhere:
- India (2nd century BC)
Philosopher Kanada proposed that all matter consists of small particles (anu). They combine to form larger structures.
- Islamic Golden Age (8th–14th century)
Scholars like Al-Razi and Avicenna debated matter and indivisibility while translating works from Greece, India, and Persia.
- Europe
Much of the Middle Ages saw Europe under scholasticism and Church teachings. In this era, Aristotle’s continuous matter theory remained dominant across Europe, excluding the Iberian Peninsula.
Key Point
Atomic thinking persisted but was largely philosophical, keeping the concept alive until experimental science could take over. During the Golden Age, atomic concepts were not experimentally tested, but they kept the atomic debate alive when Europe had almost forgotten it.
Philosophers of Islam debated atomism vs. Aristotle’s continuity in ways that directly influenced scholastic debates in Europe centuries later.

Without this transmission and refinement, there would be no Renaissance and nor would there be any intellectual soil to grow Dalton’s 1803 theory.
3. The Renaissance and Beyond (1400s–1700s)
As translated works returned to Europe via Spain and Sicily, interest in natural philosophy reignited. Scholars began questioning Aristotle’s continuous matter.
- Pierre Gassendi (France)
Gassendi revived the idea of atomism in the 17th century, blending it with Christian philosophy.
- Robert Boyle (England)
Robert was a prominent critic of Aristotle’s four-elemental idea. He proposed a “corpuscular” view of matter—tiny particles forming substances.
- Isaac Newton (England)
In Opticks, Sir Isaac Newton described matter as consisting of small, hard particles in motion.
Key Point
These thinkers prepared the intellectual ground for modern atomic science.

Reasons for Delay in Modern Atomic Theory
- Philosophy was valued over empirical testing.
- Uneven preservation of knowledge across empires.
- Aristotle’s dominance aligned with religion and common experience.
- Lack of experimental tools (microscopes and particle experiments were centuries away).
4. John Dalton (1766 – 1844)
Landing in the 18th century, meet John Dalton, a school teacher and chemist from England. Dalton brought the atom back into scientific discussion with evidence and logic.
Key Ideas
Dalton presented the first ever proper understanding of the atom, that later remembered as “Dalton’s Atomic Theory” (1803). The main postulates of the theory were:
- Matter is made of indivisible atoms. (holds inaccurate today)
- Atoms cannot be created or destroyed in chemical reactions. (holds accurate today)
- Atoms combine in whole-number ratios to form compounds. (holds accurate today)
- Atoms of the same element are identical in mass and properties. (holds inaccurate today)

Strengths
Dalton gave the atom a scientific foundation and explained the laws like conservation of mass and constant composition. His work was a bridge between chemistry and atomic theory.
Limitations
- He thought atoms were indivisible, which you now know is incorrect, and subatomic particles also exist.
- He also believed atoms are identical; however, it is not the case, as you know, isotopes also exist.
Still, Dalton’s theory provided the stepping stone others would build upon.
5. William Crookes (1832 – 1919)
By the 19th century, science had advanced enough for experiments with electricity and gases. William Crookes, a British chemist, developed a special glass tube—later called the Crookes tube—that could conduct electricity through gases at very low pressure.
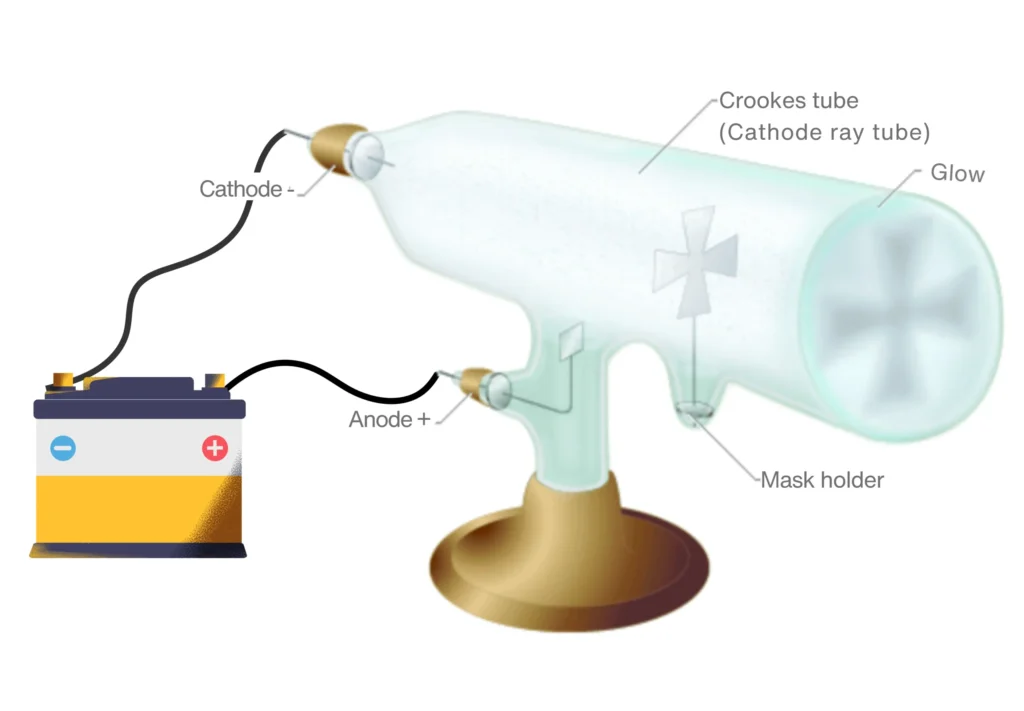
Key Discovery
In his experiment, he discovered mysterious beams that travel from the negative electrode inside the gas-filled tube and called them cathode rays.
He also demonstrated that these rays could cast shadows and move small objects, hinting at their particle-like nature.
Strengths
Though Crookes did not identify what these rays were, his experiments were a huge leap toward understanding that atoms contained smaller mobile components. He opened the door for others to dig deeper.
Limitations
At the time, scientists were still unsure if cathode rays were particles or waves. Crookes raised more questions than he answered.
6. J.J. Thomson (1856 – 1940)
Enter J.J. Thomson, who took Crookes’s mystery further. Using improved cathode ray tubes in 1897, he proved that the rays were made of tiny negatively charged particles. George Stoney coined the term “electron” for such particles. Thus, electron became the first known subatomic particle.
Key Discovery
Thomson discovered the electron and measured its charge-to-mass (e/m) ratio.
He proposed the Plum Pudding Model, according to which:
“An atom is a positively charged sphere with negatively charged electrons embedded in it.”

Strengths
Thomson shattered the belief that atoms were indivisible. He introduced the first real internal structure of the atom.
Limitations
His model could not explain the results of later experiments, particularly why electrons stayed inside the atom without flying out.
7. Eugen Goldstein (1850 – 1930)
At the end of the 19th century, a German physicist, Eugen Goldstein, was also working with modified discharge tubes having a perforated cathode. In 1886, he discovered rays travelling in the opposite direction to cathode rays. These became known as canal rays or positive rays.
Key Discovery
Goldstein suggested that atoms must contain a positive charge to balance the negative electrons. He discovered the existence of protons, positively charged particles inside atoms.

Strengths
Goldstein introduced the concept of positive charge in atoms, crucial for later models.
Limitations
He did not build a full atomic model, and his work was overshadowed by Rutherford’s later discoveries.
8. Ernest Rutherford (1871 – 1937)
Rutherford, often called the father of nuclear physics, changed everything in 1911. In his famous gold foil experiment, he bombarded thin gold foil with alpha particles. Most particles passed through, some deflected, and a few bounced back, shocking everyone.
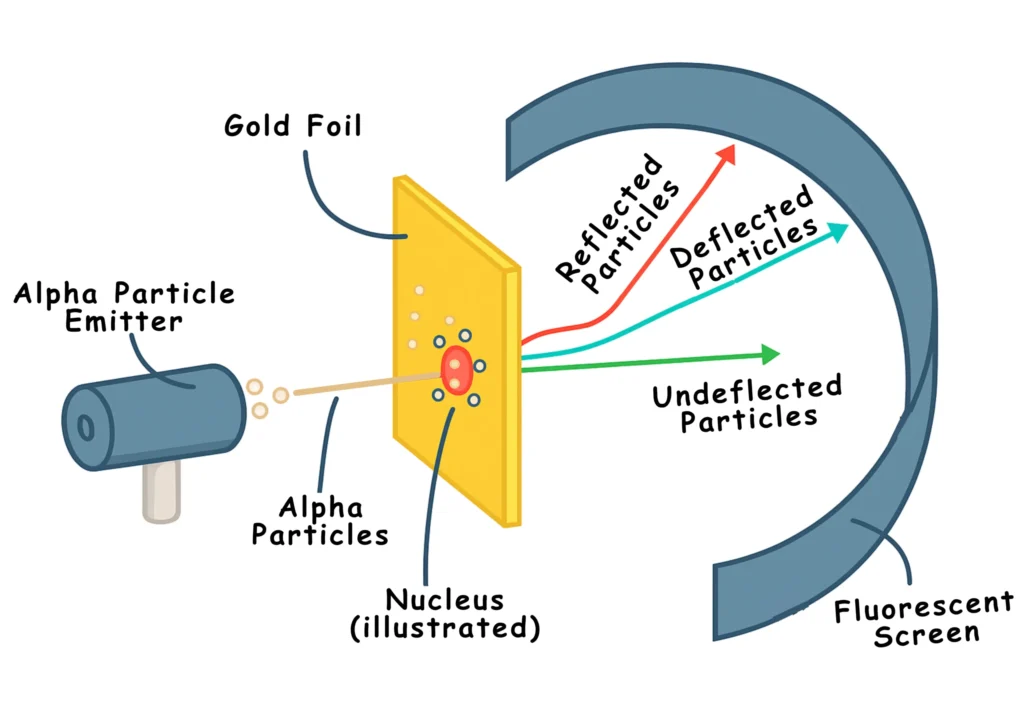
Key Discovery
The result of the experiment showed:
- Atoms are mostly space.
- Electrons orbit around the nucleus as planets around the sun.
- The orbiting electrons keep on emitting energy continuously.
- A tiny, dense, positively charged part sits at the centre called the nucleus.
The Rutherford model completely rejected Thomson’s Plum Pudding Model.

Strengths
Rutherford’s nuclear model explained scattering experiments and introduced the nucleus, a ground-breaking step.
Limitations
His model could not explain why electrons did not spiral into the nucleus due to attraction, nor could it explain atomic spectra.
9. Niels Bohr (1885 – 1962)
A student of Rutherford, Niels Bohr, refined the model of the atom in 1913 by incorporating new ideas from quantum theory.
Key Discovery
In his model, Bohr mentioned:
- Electrons orbit the nucleus in fixed paths called energy levels.
- Electrons absorb or emit energy in specific quanta when moving between levels.
- Successfully explained the bright-line spectrum of hydrogen and the stability of an atom.
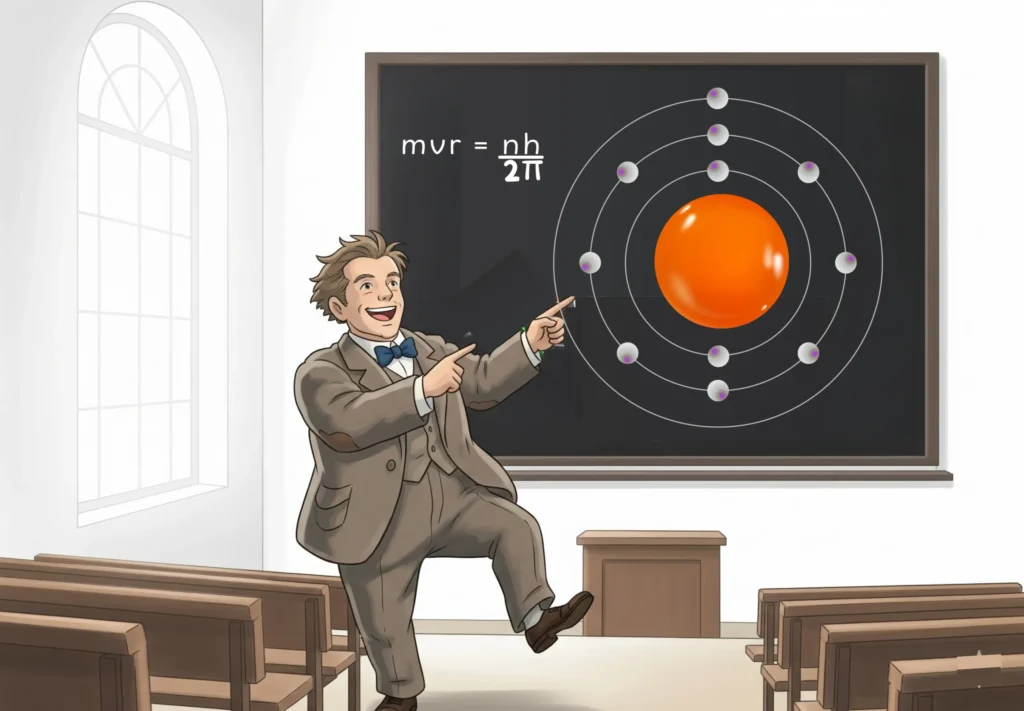
Strengths
Bohr’s theory successfully explained why electrons did not collapse into the nucleus. It matched experimental data for hydrogen and introduced quantum ideas into atomic structure.
Limitations
It failed for more complex atoms and was eventually replaced by the modern quantum mechanical model.
10. James Chadwick (1891 – 1974)
Finally, in 1932, James Chadwick completed the puzzle. He discovered the neutron, a neutral particle within the nucleus. He successfully managed to explain why the total mass of an atom was greater than just the sum of the electron and proton.
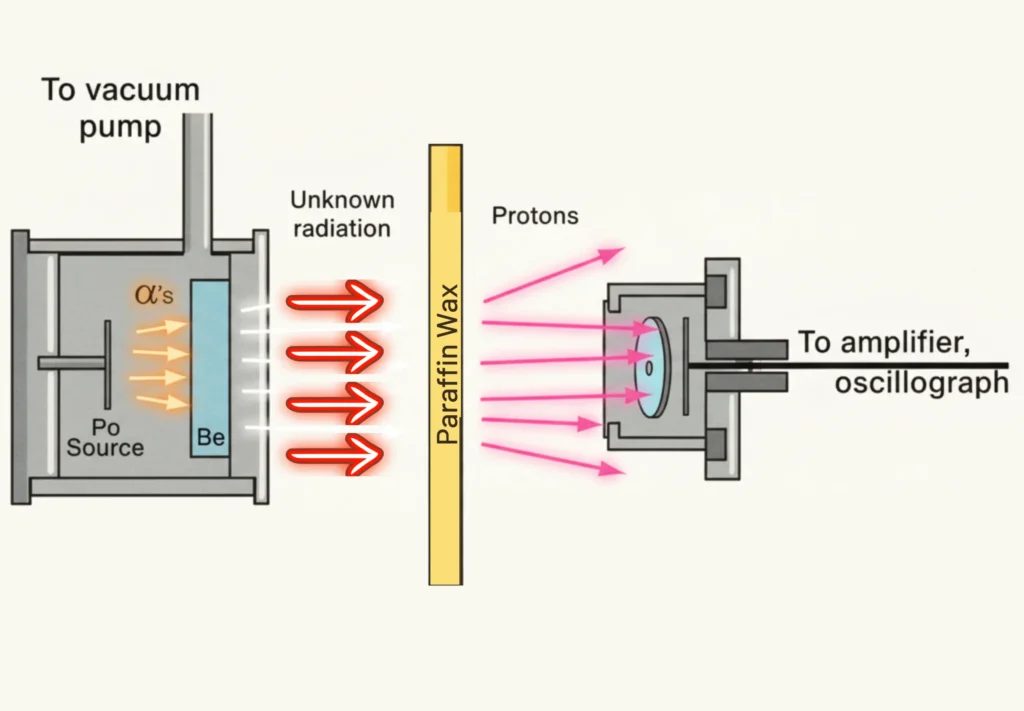
Key Discovery
The experiment with beryllium foil and alpha particles yielded the following results:
- Neutrons account for the missing mass in atoms.
- The discovery of the neutron paved the way for nuclear physics and atomic energy.
- The existence of neutrons explained why isotopes of the same element have different masses.
Strengths
With protons, neutrons, and electrons identified, the structure of an atom was complete. His discovery explained atomic stability and isotopes.
Limitations
Chadwick did not propose a new model, but his finding became the final missing piece in atomic theory.
Conclusion
The journey of the atom began with Democritus’s philosophy and evolved into the modern quantum model. From indivisible particles to a system of protons, neutrons, and electrons, the atom has become a gateway to understanding the universe.
Historical Note
- The Renaissance was not merely a “rediscovery” of Greek texts; it was the return of knowledge preserved and expanded by Muslim and Persian scholars.
- Western narratives often minimised non-European contributions and labelled Europe’s inactivity as “dark ages,” despite flourishing centres in Persia, Baghdad, Cairo, and Córdoba.
Each scientist in this long journey turned curiosity into knowledge, shaping modern science as we know it today. However, the Western narrative in terms of atomic structure is very narrow; it includes only 5-6 people.
Frequently Asked Questions (FAQs)
What do you know about the evolution of atomic theory and the structure of the atom?
The atomic theory evolved over centuries.
- Democritus proposed that matter is made of indivisible particles called atoms.
- Aristotle put forth an opposing 4 element idea (fire, water, air, and earth).
- Aristotle’s idea was later revived and debated by non-European Muslim scholars during the Golden Age.
- After the Renaissance, Robert Boyle rejected Aristotle’s ideas, supporting the corpuscular nature of matter.
- John Dalton introduced the first-ever scientific atomic theory, stating that atoms of an element are identical and combine in fixed ratios to form compounds.
- Subsequent work by Crookes, Thomson, Goldstein, Rutherford, and Bohr led to the modern atomic theory, which incorporates quantum mechanics, describing electrons in probabilistic orbitals around the nucleus.
What information was obtained from the discharge tube experiment?
The cathode ray (discharge tube) experiment showed that atoms contain negatively charged particles called electrons, which have mass and can be deflected by electric and magnetic fields. Later experiments revealed positively charged particles, later called protons.
What keeps the particles present in the nucleus intact?
The strong nuclear force binds protons and neutrons tightly in the nucleus, overcoming the electrostatic repulsion between protons.
How do electrons keep themselves away from the oppositely charged nucleus?
Electrons occupy specific energy levels or orbitals. Their motion and quantum principles prevent them from collapsing into the nucleus.
Why is it said that almost all the mass of an atom is concentrated in its nucleus?
Protons and neutrons in the nucleus are much more massive than electrons. Since electrons contribute very little mass, almost all atomic mass is in the nucleus.
Why is it needed to lower the pressure of a gas inside the discharge tube?
Lowering the gas pressure reduces collisions between gas molecules, allowing electrons to move freely and produce visible cathode rays.
What is the classical concept of an electron? How has this concept changed with time?
Classically, electrons were seen as tiny, negatively charged particles orbiting the nucleus like planets. Quantum mechanics later showed that electrons have wave-particle duality and exist in probabilistic orbitals rather than fixed paths.
What do you know about Antoine Lavoisier and Joseph Proust? How did they contribute to the modern atomic model?
Lavoisier formulated the law of conservation of mass, while Proust proposed the law of definite proportions. Their work provided the foundation for Dalton’s atomic theory, suggesting that matter is composed of discrete units (atoms) that combine in fixed ratios.
Did Democritus, Antoine Lavoisier, and Joseph Proust influence John Dalton’s work?
Yes. Democritus’ idea of indivisible atoms, Lavoisier’s conservation of mass, and Proust’s fixed ratios in compounds influenced Dalton’s atomic theory.
Who is George Stoney, and what were his contributions towards the atomic model?
George Stoney coined the term “electron” and proposed that atoms contain discrete negatively charged particles, paving the way for the discovery of the electron and the development of atomic models.