“What is a compound?” Compounds are the building blocks of everything around us. They are formed when elements combine in specific ways—guided by their valency and bonding type.
Table of Contents
Introduction
Whether it is the water you drink to quench your thirst or the salt you sprinkle on food for taste, compounds play an important role in our lives. Regardless of whether you are cooking, cleaning, or learning science—compounds are everywhere!
By understanding how they form and behave, you unlock the secrets behind everyday materials and chemical reactions. It helps us to answer the questions like:
- How water, salt, gases, etc. are made of?
- What happens in a chemical reaction?
- Why do materials act the way they do?

What is a Compound?
A compound can be defined as:
“a pure substance formed by the chemical combination of two or more different elements in a fixed ratio.”
There are 4 things to be noted:
i. Compounds are pure substances just like elements.
ii. Only chemical combination creates them.
iii. Combining elements must be of different types.
iv. They should join in a fixed ratio by mass.
Common Compounds
- Water (H₂O)
Formed by the chemical combination of hydrogen and oxygen in 1:8 by mass.
- Carbon dioxide (CO₂)
Formed by the chemical combination of carbon and oxygen in 3:8 by mass.
Note that compounds exist in all 3 states of matter i.e., solid, liquid, and gas.
Key Traits of Compounds
Two distinct traits of compounds are:
1. Different Properties than Constituent Elements
Once elements combine, the original properties of the elements are lost, and the new substance behaves differently.
Example
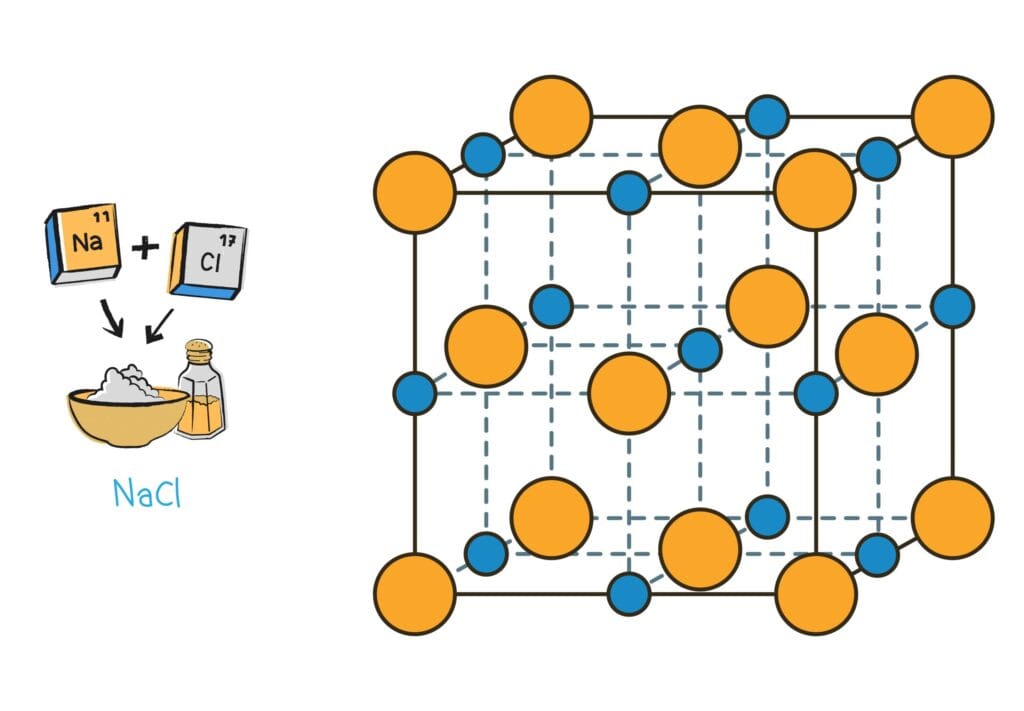
Table salt (sodium chloride – NaCl) is composed of sodium (Na) and chlorine (Cl). Here, sodium is a highly reactive metal (solid), and chlorine is a poisonous green gas (non-metal). However, when they combine, they form NaCl, which is the regular table salt—a stable, edible solid.
Na(s) + Cl(g) ![]() NaCl(s)
NaCl(s)
2. Separated by Only Chemical Methods
Compounds cannot be separated by physical methods. For instance:
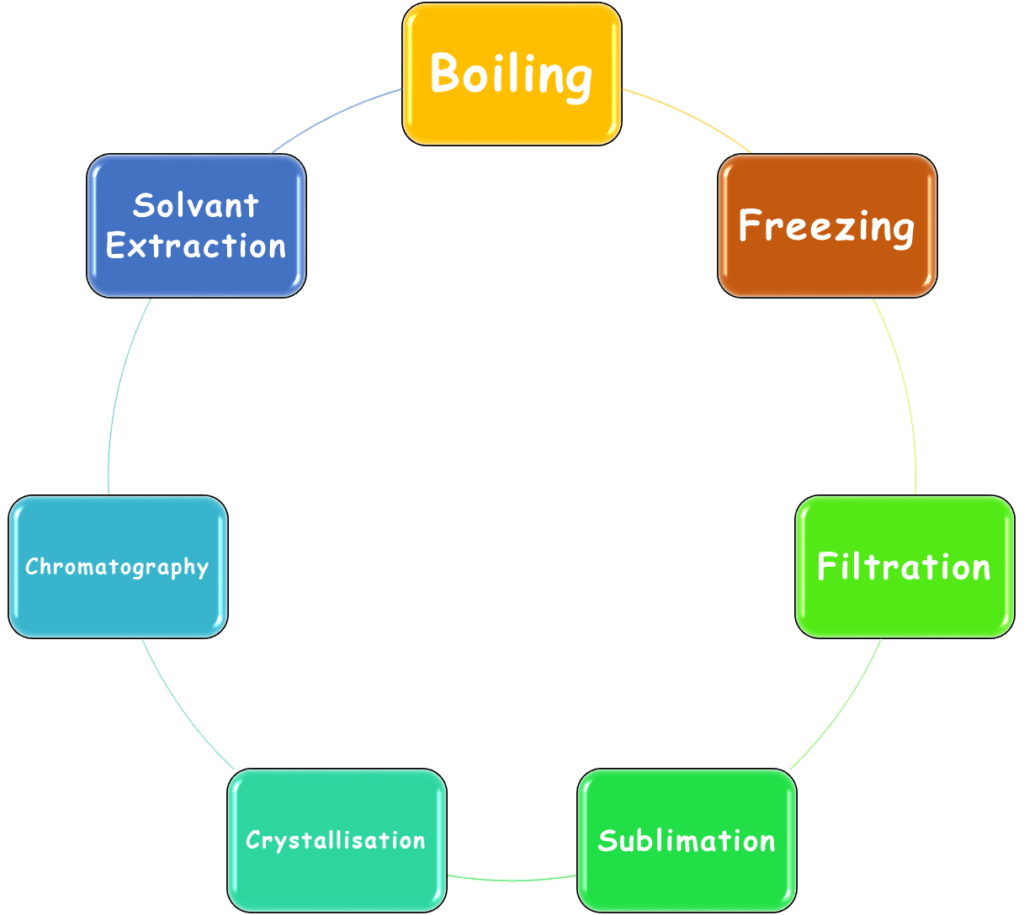
They require chemical methods for the separation of parent elements. For instance:
Example
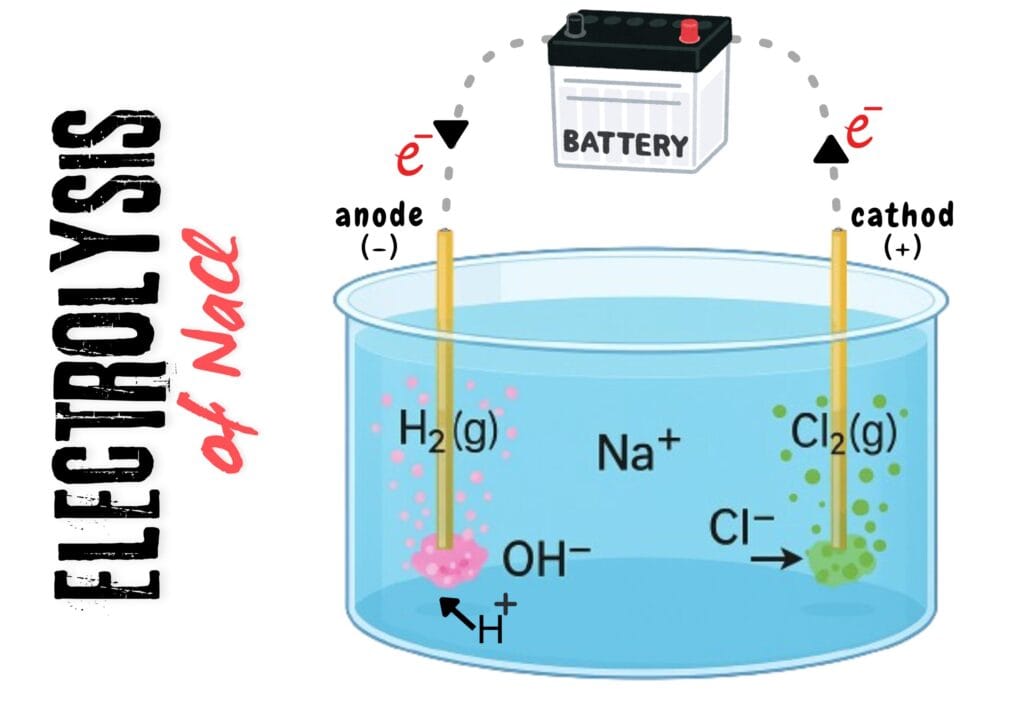
To separate table salt (NaCl) into its constituent elements – Na and Cl – physical means are not appropriate. To separate them, we need to use chemical means such as electrolysis.
Types of Compounds
Compounds can be grouped by how their atoms bond. The two main types are:
- Ionic Compounds
- Covalent Compounds
1. Ionic Compounds
Ionic compounds form when atoms transfer electrons, creating positive and negative ions that stick together.
Key Traits
- High melting and boiling points
- Crystal-like structures with strong bonds
- Often dissolve in water and conduct electricity
Examples
- Sodium chloride (NaCl)
- Copper sulphate (CuSO₄)
- Potassium iodide (KI)
2. Covalent Compounds
Covalent compounds form when atoms share electrons instead of transferring them.
Key Traits
- Exist as separate molecules
- Do not conduct electricity well
- Usually gases or liquids at room temperature
- Lower melting and boiling points than ionic compounds
Examples
- Water (H₂O)
- Methane (CH₄)
- Boron Nitride (BN)
- Silicon Dioxide (SiO2)
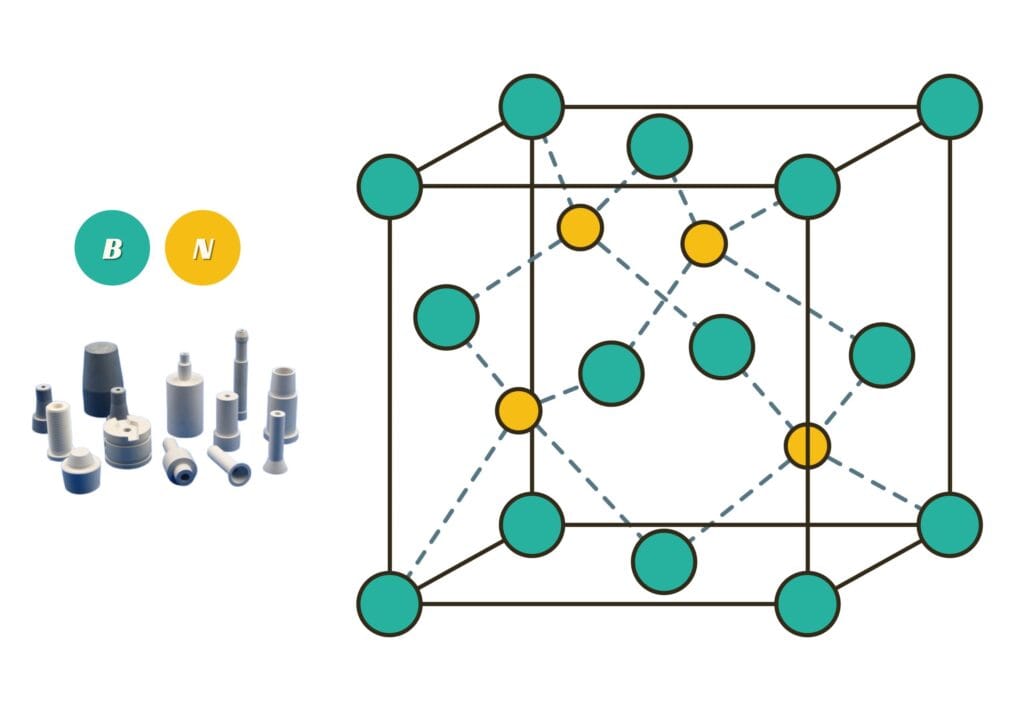
Other Types of Compounds
Besides ionic and covalent, we also find:
- Molecular compounds
- Intermetallic compounds
- Coordination complexes
3. Molecular Compounds
Compounds formed from non-metals, bonded by covalent bonds are called molecular compounds. They exist as distinct molecules.
Examples
- Water (H₂O)
- Ammonia (NH₃)
- Carbon Dioxide (CO2)
Note that all molecular compounds are covalent, but not all covalent compounds are molecular.
4. Intermetallic Compounds
These compounds are formed between two or more metals with a fixed stoichiometry and crystal structure.
Examples
- AlNi (Aluminium-Nickel)
- Mg2Cu (Magnesium-Copper)
Note, there are two important things to keep in mind for the aspirant of material science.
- All intermetallic compounds are alloys, but not all alloys are intermetallic compounds.
- These are specific, ordered compounds within the broader category of alloys.
5. Coordination Complexes
Compounds containing a central metal atom or ion (transition element) bonded to surrounding molecules or ions (ligands).
Examples
- [Fe(CN)₆]³⁻ (hexacyanoferrate)
- [Cu(NH₃)₄]²⁺ (tetraamminecopper(II))
Compounds Based on Composition and Structure
Based on composition and structure, compounds can be classified into two distinct groups:
1. Organic Compounds
These compounds contain carbon-hydrogen bonds and can include oxygen, nitrogen, sulphur, and other elements bonded to carbon.
They are usually associated with living organisms but also synthesised artificially.
Examples
- Methane (CH₄)
- Ethanol (C₂H₅OH)
- Glucose (C₆H₁₂O₆)
2. Inorganic Compounds
These compounds generally do not contain carbon-hydrogen (C-H) bonds.
Inorganic compounds are often found in non-living systems but can also be part of living organisms in the form of minerals and ions.
Examples
- Sodium Chloride (NaCl)
- Carbon Dioxide (CO₂)
- Ammonia (NH₃)
- Water (H₂O)
Why Valency Matters?
The valency of an element tells how many chemical bonds an atom can form in a compound and explains the structure and stability of molecules.
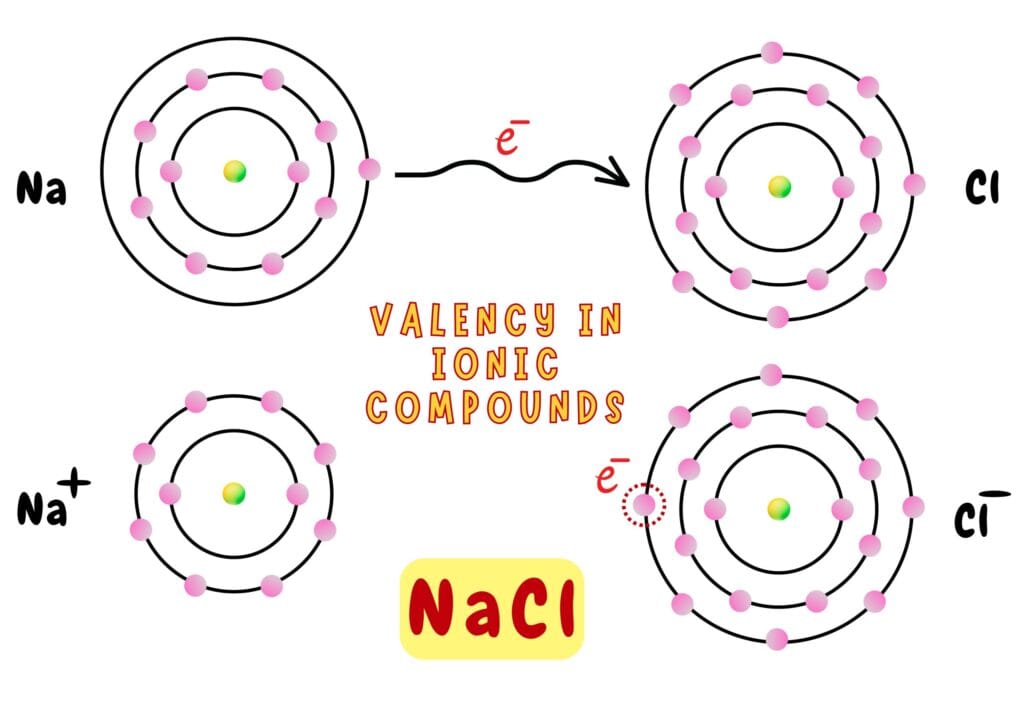
Valency in Ionic Compounds
In ionic compounds, valency refers to the number of electrons lost (for metals) or gained (for non-metals) to become stable.
Example
Sodium (Na) loses 1 electron and becomes Na⁺ (valency = 1) while Chlorine (Cl) gains 1 electron and becomes Cl⁻ (valency = 1). After combining in 1:1, they form NaCl (table salt).
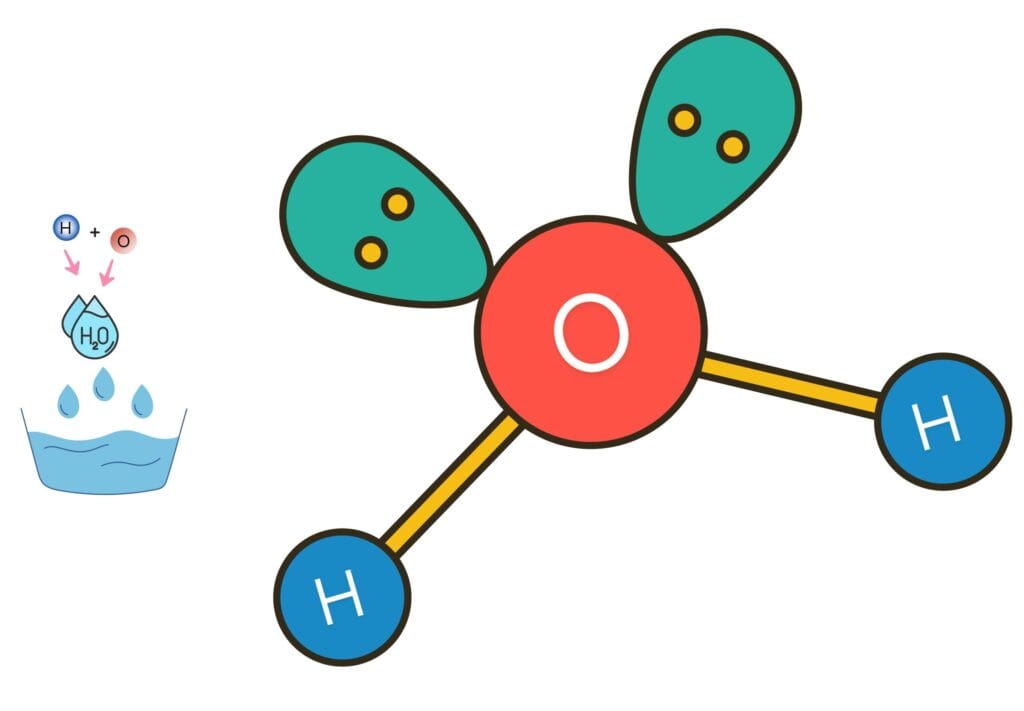
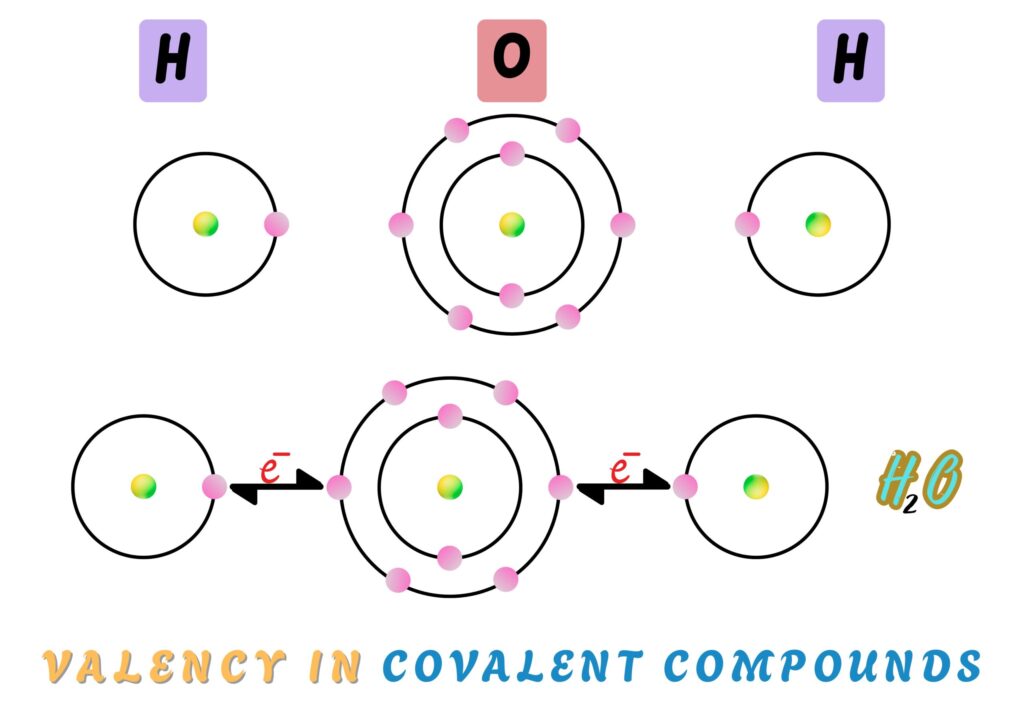
Valency in Covalent Compounds
In covalent compounds, the valency of an atom is equal to the number of bonds it forms.
Example
Oxygen has a valency of 2 while hydrogen has a valency of 1, meaning they can form 2 and 1 covalent bonds respectively.
In water (H₂O), one oxygen atom shares electrons with two hydrogen atoms.
Conclusion
Chemical compounds are everywhere and shape the world around us. Understanding how and why compounds form helps to explain:
- Chemical reactions
- Everyday materials
- Biological processes
Knowing valency and bonding allows you to predict which elements will combine—and how.
Frequently Asked Questions (FAQs)
What is a compound in simple terms?
A compound is a pure substance made when two or more different elements chemically combine in a fixed ratio by mass. Common examples include water (H₂O) and table salt (NaCl).
Which elements do the following compounds contain?
- Sugar (C₁₂H₂₂O₁₁): Carbon (C), Hydrogen (H), Oxygen (O)
- Common salt (NaCl): Sodium (Na), Chlorine (Cl)
- Lime water (Ca(OH)₂ solution): Calcium (Ca), Oxygen (O), Hydrogen (H)
- Chalk (CaCO₃): Calcium (Ca), Carbon (C), Oxygen (O)
How can you differentiate between organic and inorganic compounds?
- Organic compounds contain carbon-hydrogen (C-H) bonds and are usually found in living organisms.
- Inorganic compounds generally lack C-H bonds and are often found in minerals or non-living systems.
Are intermetallic compounds and alloys the same?
No!
Intermetallic compounds are specific, ordered combinations of metals with fixed ratios and crystal structures.
Alloys are mixtures of metals that do not have fixed compositions or ordered structures.
What are the main types of chemical compounds?
The two main types are:
- Ionic compounds (formed by electron transfer)
- Covalent compounds (formed by electron sharing)
Other types include molecular compounds, intermetallic compounds, and coordination complexes.
Can compounds be separated into their elements?
Yes!
They can be separated by chemical methods, not physical ones. For example, separating NaCl into sodium and chlorine requires electrolysis.
What is the role of valency in forming compounds?
Valency determines how many bonds an atom can form. It helps predict how elements will combine, ensuring compound formation is stable and follows specific ratios.
Are all covalent compounds also molecular compounds?
No!
While all molecular compounds are covalent, not all covalent compounds exist as discrete molecules. For instance, silicon dioxide (SiO₂) forms network structures.
What is the difference between organic and inorganic compounds?
Organic compounds contain carbon-hydrogen (C-H) bonds and are often linked to life.
Inorganic compounds usually do not have C-H bonds and are found in non-living systems or as minerals in nature.
Why do compounds have different properties from the elements that form them?
When elements combine chemically, they lose their original properties. The resulting compound has a new structure and behaves differently.
For example, reactive sodium and poisonous chlorine form safe, edible table salt (NaCl).
